Reducing Sugars Examples Disaccharides. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. All monosaccharides and some disaccharides are reducing sugars. The most important monosaccharide and reducing sugar is glucose. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Reducing sugars can be oxidized by weak oxidizing agents. In other words, disaccharides are composed by 2 sugar molecules. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: These are sugars because this group of compounds has a sweet taste as other sugars. Therefore disaccharides like sucrose are not reducing sugar.
Reducing Sugars Examples Disaccharides : Therefore Disaccharides Like Sucrose Are Not Reducing Sugar.
Spm Biology Carbohydrates Disacharides. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. The most important monosaccharide and reducing sugar is glucose. These are sugars because this group of compounds has a sweet taste as other sugars. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Therefore disaccharides like sucrose are not reducing sugar. Reducing sugars can be oxidized by weak oxidizing agents. All monosaccharides and some disaccharides are reducing sugars. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. In other words, disaccharides are composed by 2 sugar molecules. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides.
Examples of reducing disaccharides are lactose and maltose.
Some common examples of disaccharides include sucrose, lactose and maltose. In a minute, you will see an example of something that is not a reducing sugar, a disaccharide; Some prominent examples of disaccharides are lactose, sucrose, and maltose. Note that the disaccharide sucrose is not a reducing sugar. Acquired disaccharidase deficiency is relatively common, affects all disaccharides, and is if dietary modification is inconclusive or impractical, options for laboratory tests include stool analysis to identify the presence of reducing sugars in if we receive a sample containing only, for example, 0.2 g. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Glycosidic bonds form when the anomeric carbon of. To be a reducing sugar, you have to have either an aldehyde or a ketone functional group. Reducing sugars can be oxidized by weak oxidizing agents. Glucose and fructose are examples of reducing sugars. Here are some common examples of reducing sugars. Some examples of monosaccharides are glucose, fructose and galactose. Reducing sugars readily interact with amino acids and give rise to maillard reaction products, which lead to progressive browning and aroma formation. I'm only going to talk about aldehydes, but it is the same for usually when a disaccharide forms (2 glucose units, for example), the bond that links them is between the hemiacetal of the first glucose and the 4. In other words, disaccharides are composed by 2 sugar molecules. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. These are sugars because this group of compounds has a sweet taste as other sugars. 7 reducing sugars are simple sugars and include all monosaccharides and most disaccarides. An example of reducing disaccharide is maltose. All monosaccharides and some disaccharides are reducing sugars. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Types of sugar include the two main categories of sugars, monosaccharides and disaccharides. Therefore disaccharides like sucrose are not reducing sugar. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. The presence of reducing sugars or monosaccharides. Some common examples of disaccharides include sucrose, lactose and maltose. The initial rate of this reaction is dependent on the rate at which the sugar ring opens to the oxo or reducible form. A disaccharide is a type of carbohydrate that is made of two sugar units bonded together. Examples of reducing disaccharides are lactose and maltose. What color changes did you observe when you added benedict's solution to the 5% glucose solution the cloudier the solution, the higher the conc. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups.
Reducing Sugar Vs Non Reducing Sugar Acetal Hemiacetal Carbohydrate Biochemistry Mcat Youtube , There Are Certain Limitations For The Disaccharides To Give Positive Results.
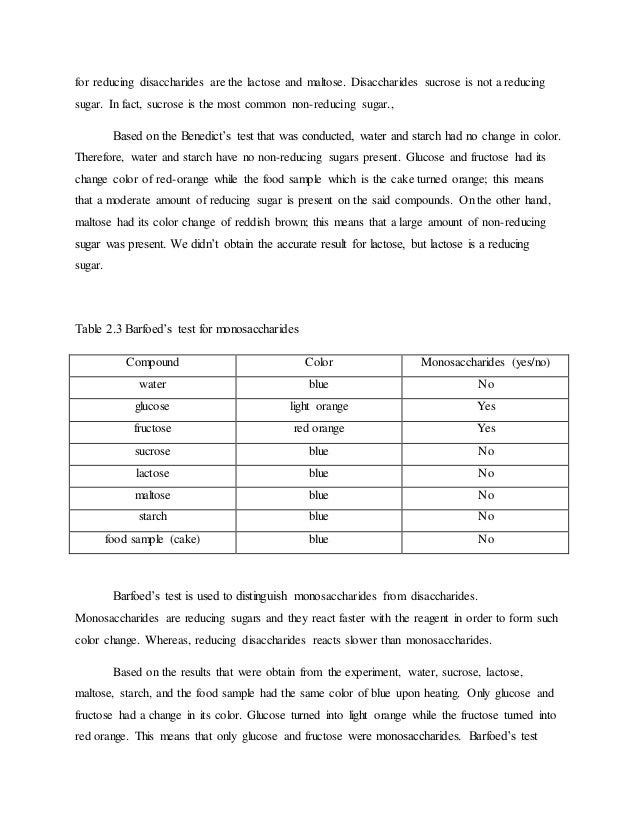
Pdf Glucose A Reducing Sugar Reducing Properties Of Sugars In Beverages And Food. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars can be oxidized by weak oxidizing agents. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. In other words, disaccharides are composed by 2 sugar molecules. Therefore disaccharides like sucrose are not reducing sugar. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. These are sugars because this group of compounds has a sweet taste as other sugars. The most important monosaccharide and reducing sugar is glucose. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides.
Reducing Sugar Wikipedia . A Reducing Sugar Is Any Sugar That Is Capable Of Acting As A Reducing Agent Because It Has A Free Aldehyde Group Or A Free Ketone Group.
Physical Chemical Properties Ppt Video Online Download. These are sugars because this group of compounds has a sweet taste as other sugars. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: All monosaccharides and some disaccharides are reducing sugars. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. The most important monosaccharide and reducing sugar is glucose. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Reducing sugars can be oxidized by weak oxidizing agents.
What Are Reducing Sugars Master Organic Chemistry - Disaccharides are a type of carbohydrate or sugar molecule.
Reducing And Non Reducing Sugars Test Ppt Download. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. These are sugars because this group of compounds has a sweet taste as other sugars. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Therefore disaccharides like sucrose are not reducing sugar. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Reducing sugars can be oxidized by weak oxidizing agents. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. In other words, disaccharides are composed by 2 sugar molecules. The most important monosaccharide and reducing sugar is glucose. All monosaccharides and some disaccharides are reducing sugars. All monosaccharides and some disaccharides are reducing sugars.
Biochemistry Chapter 7 Carbohydrates Flashcards Quizlet , Here Are Some Common Examples Of Reducing Sugars.
What Are Reducing Sugars Master Organic Chemistry. Reducing sugars can be oxidized by weak oxidizing agents. The most important monosaccharide and reducing sugar is glucose. These are sugars because this group of compounds has a sweet taste as other sugars. All monosaccharides and some disaccharides are reducing sugars. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. In other words, disaccharides are composed by 2 sugar molecules. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. Therefore disaccharides like sucrose are not reducing sugar.
Raffinose An Overview Sciencedirect Topics , Nonreducing Sugars However Do Not Have A Hemiacetal Group (An Acetal Instead) And Therefore.
How Do You Identify Reducing Non Reducing Sugar By Looking At Structure Chemistry Stack Exchange. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Therefore disaccharides like sucrose are not reducing sugar. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. These are sugars because this group of compounds has a sweet taste as other sugars. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. The most important monosaccharide and reducing sugar is glucose. Reducing sugars can be oxidized by weak oxidizing agents. In other words, disaccharides are composed by 2 sugar molecules. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. All monosaccharides and some disaccharides are reducing sugars. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules.
Chapter 4 Carbohydrate Mind Map Carbohydrates Sucrose - A Disaccharide Is A Type Of Carbohydrate That Is Made Of Two Sugar Units Bonded Together.
Di And Polysaccharides. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. These are sugars because this group of compounds has a sweet taste as other sugars. Therefore disaccharides like sucrose are not reducing sugar. Reducing sugars can be oxidized by weak oxidizing agents. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. All monosaccharides and some disaccharides are reducing sugars. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. In other words, disaccharides are composed by 2 sugar molecules. The most important monosaccharide and reducing sugar is glucose. All monosaccharides and some disaccharides are reducing sugars. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides.
In Disaccharides If The Reducing Groups Of Monosaccharides I E Aldehydic Or Ketonic Groups Are Bonded These : Glycosidic Bonds Form When The Anomeric Carbon Of.
Reducing Sugar Wikipedia. In other words, disaccharides are composed by 2 sugar molecules. All monosaccharides and some disaccharides are reducing sugars. These are sugars because this group of compounds has a sweet taste as other sugars. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. The most important monosaccharide and reducing sugar is glucose. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. All monosaccharides and some disaccharides are reducing sugars. Therefore disaccharides like sucrose are not reducing sugar. Reducing sugars can be oxidized by weak oxidizing agents. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups.
What Is The Difference Between Reducing Sugar And Starch Pediaa Com , Monosaccharides & Disaccharides Dissolve In H20 So Will Not Form A Precipiate Starch Is Not.
Bio Chapter 4 Spm. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. All monosaccharides and some disaccharides are reducing sugars. These are sugars because this group of compounds has a sweet taste as other sugars. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Reducing sugars can be oxidized by weak oxidizing agents. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. In other words, disaccharides are composed by 2 sugar molecules. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. Therefore disaccharides like sucrose are not reducing sugar. The most important monosaccharide and reducing sugar is glucose. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules.
Disaccharide Chemistry Dictionary Glossary : The Initial Rate Of This Reaction Is Dependent On The Rate At Which The Sugar Ring Opens To The Oxo Or Reducible Form.
Difference Monosaccharide Disaccharide Polysaccharide. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. The most important monosaccharide and reducing sugar is glucose. Reducing sugars can be oxidized by weak oxidizing agents. These are sugars because this group of compounds has a sweet taste as other sugars. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Two monosaccharides bond together with a glycosidic bond and form a disaccharide. In other words, disaccharides are composed by 2 sugar molecules. All monosaccharides and some disaccharides are reducing sugars. Therefore disaccharides like sucrose are not reducing sugar. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions.
Testing For Reducing And Non Reducing Sugars 2ghnvj7 Sucrose Carbohydrates - In A Minute, You Will See An Example Of Something That Is Not A Reducing Sugar, A Disaccharide;
Disaccharides Definition Function Structure Examples. The most important monosaccharide and reducing sugar is glucose. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. A reducing sugar is any sugar that is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. All monosaccharides and some disaccharides are reducing sugars. Mbd alchemie presents a video that talks about the classification of the carbohydrates as reducing and non reducing sugars subscribe to our channel: Therefore disaccharides like sucrose are not reducing sugar. All monosaccharides and some disaccharides are reducing sugars. Reducing sugars have the capacity to reduce cupric ions of benedict's or fehling solution to cuprous ions. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. In other words, disaccharides are composed by 2 sugar molecules. Reducing sugars can be oxidized by weak oxidizing agents. These are sugars because this group of compounds has a sweet taste as other sugars. Some disaccharides, such as sucrose, are nonreducing sugars, meaning they cannot donate electrons to other molecules. Two monosaccharides bond together with a glycosidic bond and form a disaccharide.